Rapid SARS-CoV-2 Antigen Test Card
- FDA EUA certificate
- Room temp storage
- Multiple packages
- Easy to use
- Result in 15 minutes
- For users above age 2
Test Procedure Overview
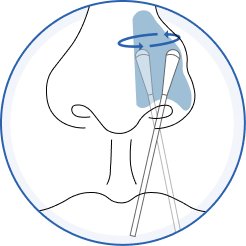
1.Swab
Slowly rotate the swab in a circular motion 5 times by firmly pressing against the inside walls of the nostril for a total of 15 seconds. Do not just spin the swab. Gently remove the swab and repeat in the other nostril using the same swab.
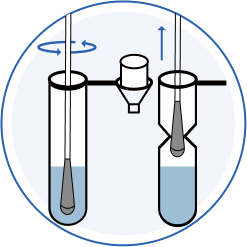
2.Dip
Place swab in buffer tube. Rotate swab 5 times. Set a timer and leave swab in buffer tube for 1 minute. Pinch buffer tube with fingers and remove the solution from swab as much as possible.
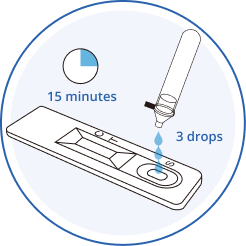
3.Drop
Invert the buffer tube and add 3 drops of test sample into the sample well (S) by gently squeezing the extraction tube. Do not add test sample to the rectangular results window.

4. See Results
If a Control (C) line and the Test (T) line are visible, the test is positive. Any faint visible pink/purple test (T) line with the control line (C) should be read as positive. If a control line (C) is not visible, even if the test line is visible, the result must be considered invalid.
Instruction Video
Order Information
Documents
Contact Us
Tel:
+1-800-689-7794 (technical support)
Company Info:
Address: 90-94 Tianfeng Road, Jimei North Industrial Park Xiamen, Fujian 361021, China
Product Inquiry
Frequently Asked Questions
The test kits that you received have been approved by the FDA and are free of charge. If you no longer wish to receive this test kits, please contact the supplier that sent you the test cards to discontinue delivery. We investigated and found that Medicare patients will receive eight test cards per month in the period of Public Health Emergency. It is our understanding that Medicare patients will stop getting test cards at the end of Public Health Emergency (October 2022). Please note that the Public Health Emergency could be extended.
A negative test means that the virus that causes COVID-19 was not detected in your sample. It is unlikely that you have COVID- 19. However, even if your test is negative, continue to observe all hygiene and safety measures. If you suspect that you have an infection (i.e., if you have prolonged symptoms or if your symptoms are worsening), contact your doctor/primary care physician. You may have another infection, or your test result may be false. Negative results do not rule out COVID-19. This means that you could still have COVID-19 even if your test is negative. If you do not have symptoms of COVID-19 and your result is negative, you should test again with at least 24 hours and no more than 48 hours between tests.
A positive test result means that the virus that causes COVID-19 was detected in your sample, and it is very likely that you have COVID-19.Please contact your doctor/primary care physician or your local health authority immediately and adhere to the local guidelines regarding self-isolation. Your doctor may require you to undergo a molecular PCR test to confirm the result. There is a very small chance that this test can give a result that is incorrect (a false positive).
The test is invalid. The test is not working correctly and you should perform another test using a different test kit. You may have performed the test incorrectly. Carefully read the Quick Reference Instructions and repeat the test. If your test result is still invalid, please contact a doctor or visit a COVID-19 test center.
News
Boson Introduction
Xiamen Boson Biotech Co., Ltd., as a specialist of in vitro diagnostic kits field was founded in 2001, develops and manufactures high-quality point of care and other immunoassay test kits for the world-wide market. Our factory is operated strictly under ISO 13485 and GMP guidelines. Our product lines provide immunoassays in various formats to detect infectious diseases, cardiac markers, drugs of abuse, fertility hormones and tumor markers. Many of our products have been approved by the China NMPA and are CE certified.

- This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA;
- This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens; and,
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
- For more information on EUAs visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatoryand-policy-framework/emergency-use-authorization
- For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19

